
Reports of BIA-ALCL linked to Textured Implants
Millions of women have opted for textured implants when undergoing breast augmentation or breast reconstruction. Textured implants were designed over 50 years ago to prevent or minimize the development of scar tissue and decrease the likelihood of capsular contracture. For this reason, many women and surgeons alike favored this style of implant, especially in breast reconstruction cases where there is a heightened risk of capsular contracture.
FDA Warns Textured Implants Could Increase Risk of Cancer
Unfortunately, recent studies have linked textured implants to breast implant associated anaplastic large cell lymphoma (BIA-ALCL). In October of 2021, the FDA released an article regarding strengthened safety requirements pertaining to breast implants. The article included studies and updated information about the increased risk of BIA-ALCL in patients with textured implants.
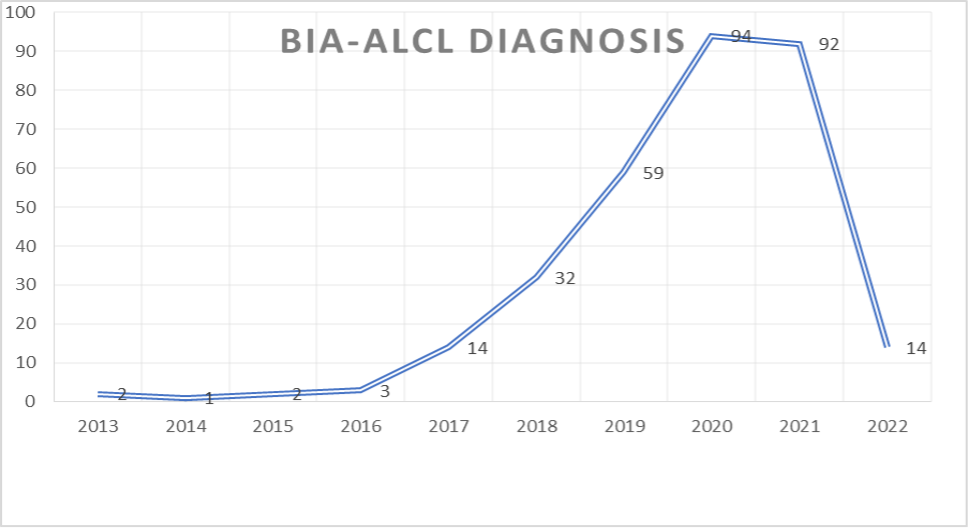
Diagnosed BIA-ALCL Cases by Year
The graph above depicts an alarming increase in BIA-ALCL diagnoses in recent years. Of those receiving a positive diagnosis, 90.4% had an Allergan implant, 7.3% had a mentor implant, 1.5% had a Sientra implant, and the remaining .09%were from various other breast implant manufacturers.
Currently, the Food and Drug Administration has not recommended removal of textured implants if you are not experiencing any symptoms. However, many women are opting to have them removed or replaced with smooth implants out of fear of developing cancer for a first, second or even third time.
You should discuss any concerns you have of developing BIA-ALCL with your doctor and/or get tested for CD30 before surgical intrusion.
It is important to note that BIA-ALCL is not breast cancer. BIA-ALCL is a type of blood cancer that is commonly spread through breast tissue and lymph nodes. It typically takes around 7-10 years to develop. Common symptoms of BIA-ALCL include:
- A change in the shape of the implant or the breast
- The development of breast asymmetry
- Increased firmness in the breast
- Pain in or around the breast
- The development of lumps around the breast or armpit
- Itching or redness around the breast
- Swollen lymph nodes
- Development of fluid around the implant
- Lesions
- Fever
- Fatigue
- Night sweats
If these symptoms are present patients will typically receive an ultrasound or MRI to check for fluid or lumps around the implant. If either are present the patient must then undergo a biopsy where the fluid or tissue is tested for CD30. A CD30-positive test result indicates the presence of hematopoietic malignancies (blood cancer).
If you or a loved one have been diagnosed with BIA-ALCL after a breast implant surgery, you do not have to go through this process alone. The attorneys at The Yost Legal Group are currently investigating and pursuing claims for women who have been diagnosed with BIA-ALCL after receiving Allergan Biocell textured breast implants. Call us today at 1 (800) YOST – LAW or email us at info@yostlaw.com for a free consultation. We will investigate the circumstances of your individual case, answer any questions you have, and fight to get you the compensation you deserve.