New Warning Labels for Non-Steroidal Anti-Inflammatory Drugs (NSAIDS)
In the wake of new research indicating increased risks of heart attacks and strokes, the FDA is requiring new warning labels for nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen, which for years have been viewed largely as reliable, over-the-counter solutions to a myriad of common health problems. On July 9th, 2015, the Food and Drug...
CONTINUE READING
Defective Medical Devices Can Have Devastating Consequences
Manufacturers of medical devices have an obligation to ensure the safety of their products. The effects of a faulty device can be devastating. Companies that fail in their duty to keep patients safe must be held fully accountable. If you received a hip replacement from the Stryker Orthopedics Corporation, you could be entitled to compensation....
CONTINUE READING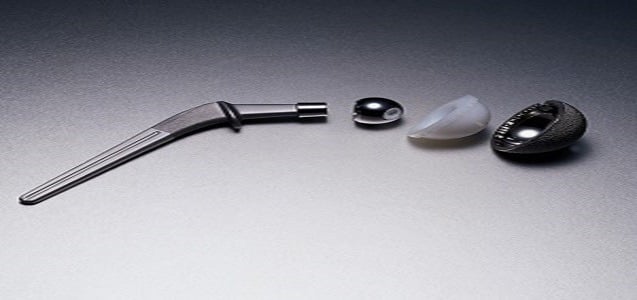
Hip Replacement Manufacturer To Pay Over $1 Billion in Major Settlement
Stryker Corp, a major manufacturer of artificial hip implants, announced on Monday, November 3rd that it has reached a settlement agreement with the thousands of patients that were harmed by their all-metal Rejuvenate and ABG II hip-replacement models. Stryker, a subsidiary of the DePuy division of Johnson & Johnson, originally recalled these models in July...
CONTINUE READINGFDA’s Generic Drug Warning Labeling Rule Change
The New York Times recently published an editorial in support of the FDA’s generic drug labeling rule. AAJ (American Association for Justice) released an editorial board memo on generics and Katie Gommel (AAJ Press Secretary) did an outstanding job working behind the scenes with Public Citizen to shape this editorial. Late last year, the Food...
CONTINUE READINGFDA to Review Rules for Over-the-Counter Drugs
The FDA has launched a review of the way it determines the safety of over-the-counter drugs such as acetaminophen. Current regulations allow the manufacturers of over-the-counter medications to drag their feet when it comes to updating warning labels. The process of increasing the warning on a potentially dangerous drug can take years, during which time hundreds,...
CONTINUE READINGGeneric Drug Manufacturers Oppose FDA Label Proposal
The FDA has proposed label changes that would require generic drug manufacturers to update warning labels when new safety concerns come to light, even if those label changes have not first been made by the original manufacturer of the medication. The Generic Pharmaceutical Association, a trade group representing generic drug manufacturers, claims the proposed changes...
CONTINUE READING