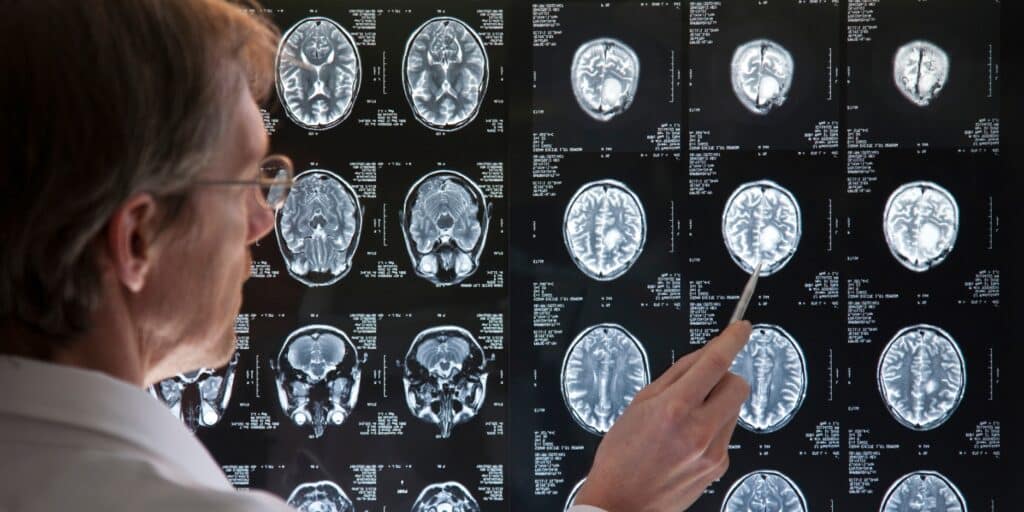
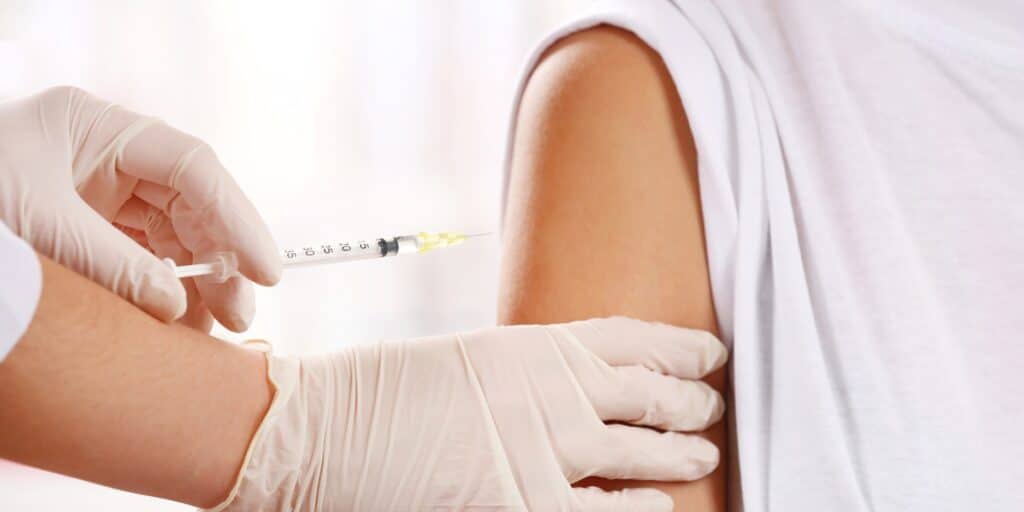
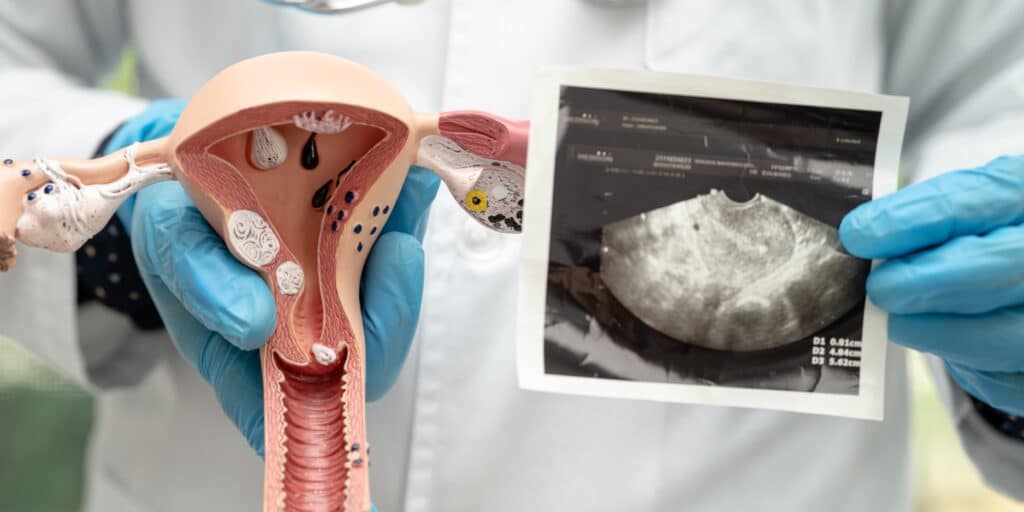
A new study raises concerns about the potential link between Depo-Provera® contraceptive injections and meningioma brain tumors. Research suggests women who have used Depo-Provera® long-term may face an increased risk of developing this condition. Do you qualify to file a Depo Provera lawsuit?
If you or someone you love has been diagnosed with a meningioma after using Depo-Provera®, you may qualify to file a Depo Provera lawsuit. Let us help you seek the justice and financial compensation you deserve.
Contact The Yost Legal Group today at 800-967-8529 for a free consultation. Our experienced Depo attorneys are ready to help you explore your legal options. Don’t wait—call now.
Know Your Rights After Depo-Provera Meningiomas Diagnosis
- Prolonged Use of Depo-Provera® May Increase Risk of Brain Tumors
- Recent research highlights an important concern for women using Depo-Provera® as a contraceptive. Studies have shown that extended use of Depo-Provera® significantly increases the risk of developing meningioma, a type of brain tumor.
- 5.6x Higher Risk with Depo-Provera Women who use Depo-Provera® for more than one year face a 5.6-times higher risk of developing meningiomas, according to a study in the British Medical Journal. If you’ve experienced health complications linked to Depo-Provera®, seek guidance today.
- Meningiomas May Require Surgical Treatment – Meningiomas are tumors that can grow and put dangerous pressure on the brain. These growths often demand invasive surgery, which comes with serious health risks and potential complications. If you or a loved one are facing a diagnosis, consult a healthcare specialist immediately for guidance and treatment options. Early intervention is critical.
- Common Symptoms of Meningioma: Persistent headaches, vision issues, seizures, hearing loss, or weakness in the limbs could be symptoms of a meningioma—a type of brain tumor. These signs should not be ignored. If you’re experiencing any of these symptoms, consult a healthcare professional immediately for evaluation. Early detection can make all the difference.
- Protect Your Right to Compensation: If you’ve developed a meningioma after using Depo-Provera®, you could be eligible for compensation. This may cover medical expenses, lost wages, pain and suffering and more.
- Act Quickly to Protect Your Rights – Statutes of limitations impose strict deadlines. Delaying action could jeopardize your legal claim.
- Get a Free Consultation Today – Contact us now to confidentially discuss your case and explore your legal options with our experienced team.
Possible 5.6x higher risk of meningioma with Depo-Provera® use.
Recent Study Links Depo-Provera® to Meningioma
Depo-Provera® is a hormonal birth control method delivered via injection every three months. It contains medroxyprogesterone acetate, a synthetic form of progesterone, and works by preventing ovulation and changing the uterine lining to reduce the chances of pregnancy.
Key Information About Depo-Provera® You Need to Know
- Purpose: Depo-Provera® is a trusted, long-lasting contraceptive designed to prevent pregnancy effectively.
- Administration: It’s administered through an intramuscular injection in the arm or buttock every 12 weeks, ensuring consistent protection.
- Effectiveness: When used as directed, it is over 99% effective in preventing pregnancy, offering reliable peace of mind.
- Popularity: This method is favored by millions of women globally for its convenience and highly dependable results.
A recent study published in the British Medical Journal in March 2024 revealed concerning findings about Depo-Provera®, a widely trusted contraceptive.
The study indicates a potential link between prolonged use of Depo-Provera® and an increased risk of brain tumors, specifically meningiomas.
Women who have used Depo-Provera for over a year may face a significantly higher risk of developing these tumors. If you or a loved one has been diagnosed with a brain tumor after using Depo-Provera®, it is crucial to act now.
Contact The Yost Legal Group to learn more about the Depo Provera litigation. Contact us for a FREE, no-obligation consultation today.
The experienced defective products attorneys at The Yost Legal Group are here to support you. We are helping women across the U.S. file dangerous drug lawsuits and seek justice. Call us today at [Phone Number] for a free consultation. Don’t wait—your health and rights matter.
Key Findings on Depo-Provera® and Meningioma Risk
A new study reveals significant findings about the connection between Depo-Provera® and intracranial meningiomas. Here’s what you need to know:
- Higher Risk Identified: Women who used Depo-Provera® for over a year faced a 5.6x increased risk of developing intracranial meningiomas compared to women who did not use it.
- Reliable Data: The analysis was conducted on medical records from over 100,000 women through the French National Health Data System.
- Long-Term Use Concerns: Risks were significantly higher among women who used Depo-Provera® for extended periods, pointing to potential dangers with prolonged use.
- Specific to Depo-Provera®: Other progestogens, such as progesterone or levonorgestrel intrauterine systems, did not show an increased risk, underlining a unique concern with Depo-Provera®.
Key Implications of the Depo Side Effects Findings
- Substantial Risk Increase: The study reveals a 5.6-fold increase in the risk of meningioma linked to the use of Depo-Provera®, underscoring a significant and alarming association.
- Prolonged Use is Riskier: Long-term users face an escalated risk, highlighting the critical role duration plays in user vulnerability.
- Insufficient Awareness: Many women may have been exposed to this elevated risk without proper disclosure or warnings.
- Call for Immediate Regulatory Action: These findings signal an urgent need for regulatory agencies to reassess the safety of Depo-Provera®, implement additional warnings, and evaluate potential restrictions.
Your Legal Rights After a Meningioma Diagnosis
Pharmaceutical companies must prioritize the safety of their products and fully inform consumers about any potential risks associated with their drugs.
If you’ve been diagnosed with a meningioma after using Depo-Provera®, understanding Pfizer Inc.’s responsibilities as the drug’s manufacturer is key.
Failures to identify and disclose the risk of meningiomas early in Depo-Provera’s market lifecycle may have caused significant harm to consumers. It’s critical to know your legal rights and take action now.
Filing a Depo Provera lawsuit is the first step toward seeking justice. Contact The Yost Legal Group at 1-410-659-6800 for a free consultation.
Our experienced Depo Provera lawyers are here to provide guidance, support, and fight for justice on your behalf. Don’t wait—your path to justice could begin today.
Potential Failure to Properly Test the Product
- Insufficient Research: There may have been a lack of thorough studies to assess long-term neurological effects.
- Ignored Warnings: Early reports of adverse effects might have been overlooked, raising concerns about due diligence.
Possible Failure to Provide Adequate Warnings
- Missing Critical Information: The product labels may not properly highlight the increased risk of meningioma, leaving users uninformed.
- Misleading Representations: Marketing efforts could prioritize benefits while downplaying serious risks, potentially putting consumers at risk.
Market Effects of Depo-Provera®
- Failure to Report Adverse Events: Evidence suggests a possible delay in the timely reporting of meningioma cases linked to the use of Depo-Provera®.
- Insufficient Action on Emerging Evidence: Reports point to a lack of prompt updates to warnings or further research being conducted to address the newly identified risks.
The Yost Legal Group Will Protect Your Rights
If you or a loved one have experienced adverse effects, you may have a claim against the drug manufacturer. Contact The Yost Legal Group at 1-410-659-6800 for a free consultation. Our trusted team is here to provide guidance and advocate for your rights. We will file a Depo Provera lawsuit on your behalf.
Free case evaluations are now available for women who developed meningioma after using Depo-Provera®. Contact us today to explore your legal options and seek the justice you deserve.